Solid oxide electrolysis cell (SOEC) can convert CO2 and H2O simultaneously into syngas or hydrocarbon fuel at the cathode, and produce high purity O2 at the anode. It has the advantages of solid and modular structure, fast reaction rate, high energy efficiency, and low cost. Therefore, SOEC has shown great potential application prospects in CO2 conversion and renewable clean electricity energy storage.
Perovskite-type oxides have received extensive attention in the fields of catalysis and energy due to their excellent doping ability, carbon deposition resistance and redox stability. However, compared with traditional nickel-based cathode, the application of perovskite electrodes is limited due to their insufficient electrocatalytic activity. Doping active components into the perovskite bulk, and then exsolving metal nanoparticles in reducing atmosphere to construct highly active and stable metal/perovskite interfaces is an effective way to improve the electrochemical performance of perovskite-based catalysts. However, the exsolution of metal nanoparticles still has challenges, such as low particle density, large particle size, etc. In addition, the formation mechanism and catalytic mechanism of the metal/perovskite interfaces still need to be clarified using in situ dynamic characterizations.
Recently, a research team led by Prof. WANG Guoxiong and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) collaborated with the senior engineer Hiroaki Matsumoto and Chaobin Zeng from Hitachi Hightechnology Company have made new progress in the research of high temperature CO2 electrolysis in SOEC. Redox cycle manipulations promoted the exsolution of high-density metal/perovskite interfaces, which significantly improved the CO2 electrolysis performance and stability.
This study was published in Nature Communications on September 27.
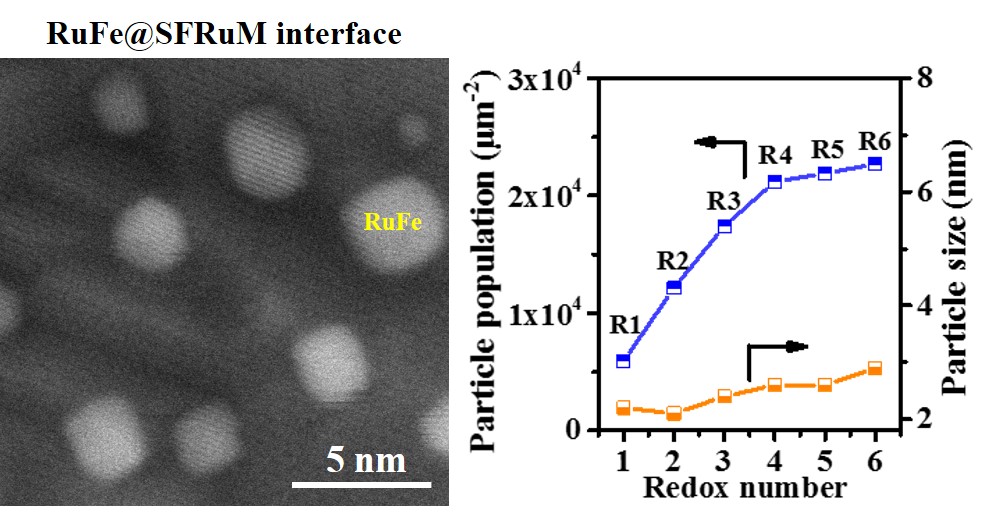
The researchers prepared Ru-doped Sr2Fe1.4Ru0.1Mo0.5O6-δ (SFRuM) double perovskite. The repeated redox manipulations promoted the exsolution of RuFe alloy nanoparticles from 5900 μm-2 (R1) to 22680 μm-2 (R6), where the mean particle size is between 2.2 and 2.9 nm, thus effectively regulated the density of the RuFe@SFRuM interfaces. Combined with in situ atmosphere electron microscopy, elemental maps and electron energy loss spectroscopy, the formation and regeneration mechanism of RuFe@SFRuM interface under reducing and oxidizing atmosphere were revealed. The enrichment in Ru species on the surface was proposed to promote the exsolution of high-density RuFe@SFRuM interfaces.
In situ atmosphere electron microscopy, electrochemical impedance spectroscopy combined with density functional theory calculations confirmed that the RuFe@SFRuM interface promoted CO2 adsorption and activation. Compared with the SFRuM cathode, the RuFe@SFRuM cathode has a 74.6% increase in current density for CO2 electrolysis at 1.2 V and 800 °C, and exhibits a high stability of CO2 electrolysis for 1000 h.
“This work presents a new strategy for achieving efficient and stable CO2 electrolysis in SOEC,” said Prof. WANG.
The above work was supported by the National Natural Science Foundation of China, the National Key Research and Development Program, and the Youth Innovation Promotion Association of the Chinese Academy of Sciences. (Text/Picture Houfu Lv and Le Lin).