Electrocatalytic CO2 reduction reaction (CO2RR) converts CO2 and water into C2+ products such as ethylene and ethanol under mild conditions, which realizes carbon recycling and storage of renewable energy simultaneously. Copper can convert CO2 into C2+ fuels and chemicals due to the moderate adsorption of *CO intermediates. However, the Faradaic efficiency is still limited at high current densities, especially in a practical zero-gap electrolyzer using a gas diffusion electrode and anion exchange membrane (MEA-based electrolyzer). Electrochemical in situ reconstruction of CuxO and other Cu derivatives has been extensively investigated as an efficient strategy to construct nanostructured Cu catalysts with defects and low-coordinated sites, which greatly promotes C-C coupling during CO2RR.
Recently, a research team led by Prof. Guoxiong Wang and Prof. Xinhe Bao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) designed an electrochemically reconstructed copper pyrophosphate (Cu2P2O7) catalyst and achieved efficient production of C2+ chemicals in CO2RR. This study was published in Angewandte Chemie International Edition on December 2, 2021.
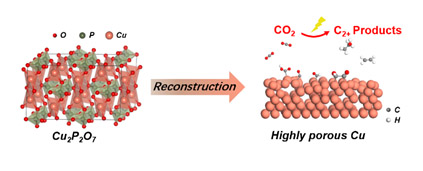
An electrochemically reconstructed Cu2P2O7 catalyst achieved efficient production of C2+ chemicals in CO2RR
The researchers introduced phosphorus into the lattice of cupric oxide (CuO) to synthesize Cu2P2O7 with a high density of grain boundaries. The catalyst was electrochemically reconstructed under CO2RR conditions, thus obtained a highly porous Cu with a high electrochemically active surface area (ECSA), abundant defects and low-coordinated sites. The reconstructed Cu2P2O7 catalyst achieved a Faradaic efficiency of 73.6% for C2+ products at an applied current density of 350 mAcm-2 in a MEA-based electrolyzer, with ethylene Faradaic efficiency of 39.8% and ethanol Faradaic efficiency of 23.8%, remarkably higher than the CuO counterparts. In situ Raman spectroscopy and density functional theory (DFT) calculations revealed that the defects and low-coordinated sites from reconstructed Cu2P2O7 catalyst provided a suitable condition for bridge and atop adsorbed *CO, which was more favorable for C-C coupling, thus resulted in an improvement of C2+ production.
The above work was supported by the National Key R&D Program, the National Natural Science Foundation, Dalian National Laboratory for Clean Energy (DNL180404, DNL201924), the Strategic Priority Research Program of the Chinese Academy of Sciences, the CAS Youth Innovation Promotion and the fellowship of China Postdoctoral Science Foundation. (Text by Jiaqi Sang and image by Tianfu Liu)
Article link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202114238