Uncovering how hydrogen transfers and what factors control hydrogen conductivity on solid surface is essential for enhancing catalytic performance of H-involving reactions, which is however hampered due to the structural complexity of powder catalysts, in particular, for oxide catalysts.
Recently, a research team led by Prof. Rentao Mu and Prof. Qiang Fu from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) direct observed the acceleration of hydrogen spillover via surface-lattice-confinement effect. This work was published in Nature Communications on February 04, 2023. (Article link: https://doi.org/10.1038/s41467-023-36044-8)
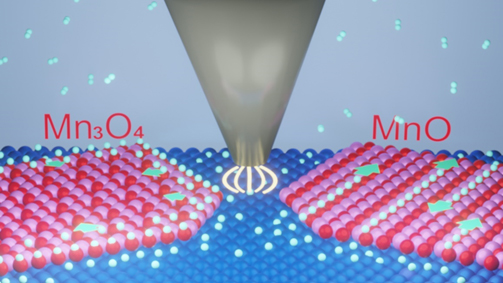
Stripe-like MnO(001) and grid-like Mn3O4(001) monolayers were constructed on Pt(111) substrate and hydrogen spillover was investigated atop. Atomic-scale visualization demonstrates that hydrogen species from Pt diffuses unidirectionally along the stripes on MnO(001), whereas it exhibits an isotropic pathway on Mn3O4(001). Dynamic surface imaging in H2 atmosphere reveals that hydrogen diffuses 4 times more rapidly on MnO than the case on Mn3O4, which is promoted by one-dimension surface-lattice-confinement effect. Theoretical calculations indicate that a uniform and medium O-O distance favors hydrogen diffusion while low-coordinate surface O atom inhibits it. Our work illustrates the surface-lattice-confinement effect of oxide catalysts on hydrogen spillover and provides a promising route to improve the hydrogen spillover efficiency.
This work was financially supported by National Key R&D Program of China (2021YFA1502800), National Natural Science Foundation of China (No. 91945302, No. 21825203, No. 22288201, and No. 22272162), Photon Science Center for Carbon Neutrality, LiaoNing Revitalization Talents Program (XLYC1902117), and the Dalian National Laboratory for Clean Energy (DNL) Cooperation Fund (DNL201907). (Text/image by Rentao Mu/Yijing Liu)