Sintering of metal nanocluster catalysts under harsh reaction conditions poses a significant challenge in heterogeneous catalysis, often resulting in poor stability. Utilizing spatial confinement and strong metal-support interactions can constrain metal nanoclusters, allowing them to remain stable at high temperatures at the expense of catalytic activity. Breaking this activity-stability trade-off for metal nanocluster catalysts is crucial for their practical applications.
Recently, a research group led by Prof. BAO Xinhe and Prof. FU Qiang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) introduced a dynamic confinement method to stabilize active silver nanoclusters in CO oxidation. This study was published in Angewandte Chemie International Edition.
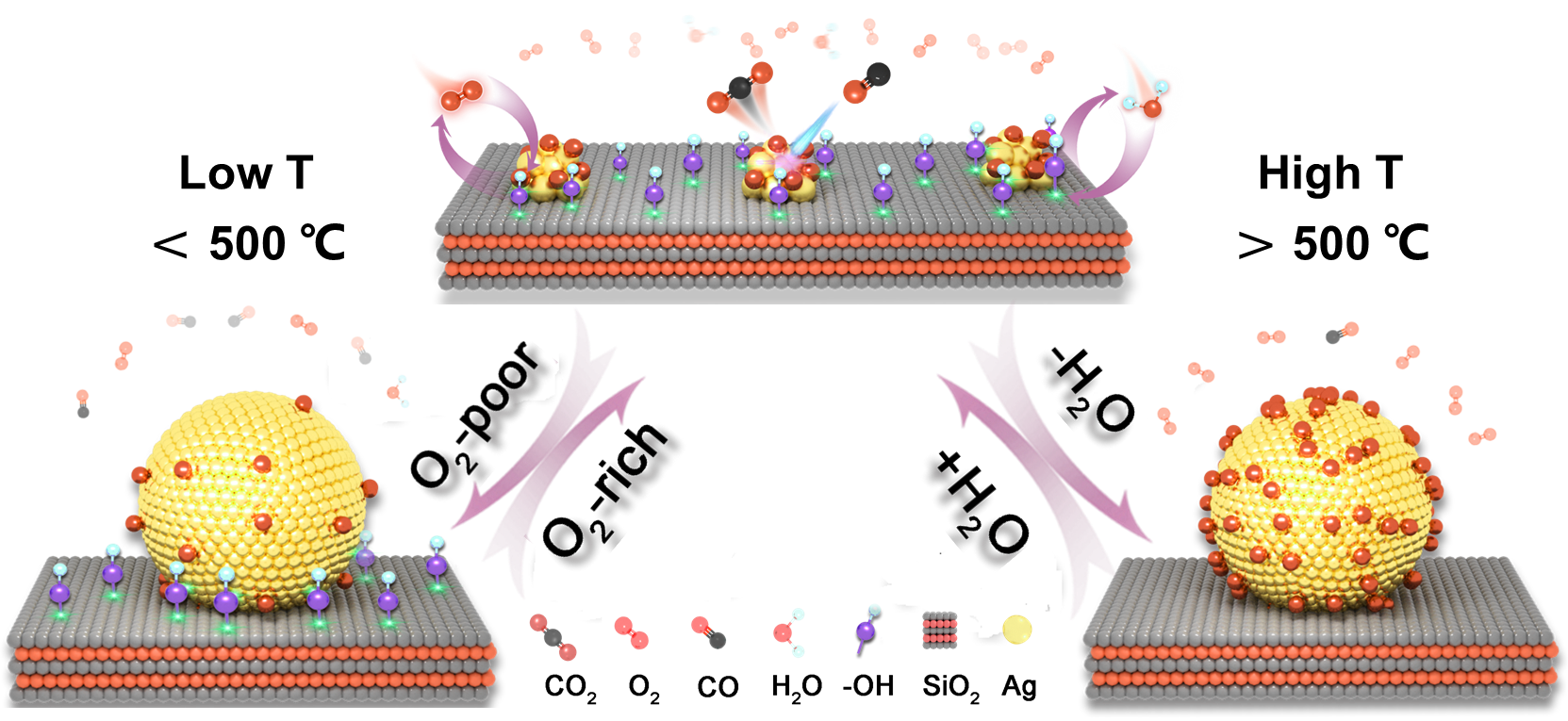
Dynamically confined active silver nanoclusters by reaction environment with ultrawide operating temperature window in CO oxidation (Image by Li Rongtan)
The researchers demonstrate that the activity-stability trade-off can be broken through rational design of surrounding microenvironment of the supported nanocatalyst including gas adsorbate overlayer and underneath support surface chemistry. The study reveals that chemisorbed oxygen species on Ag surface and surface hydroxyl groups on oxide support, which are dynamically consumed during reaction but sustained by reaction environment (O2 and H2O vapor), drive spontaneous redispersion of Ag particles and stabilization of highly active Ag nanoclusters.
Such a dynamic confinement effect from gas-catalyst-support interaction enables the Ag nanoclusters to exhibit complete catalytic oxidation of CO over a wide temperature window as well as remarkable sintering-resistance at 300 °C over 1000 h.
"Adsorbates on metal surface and OH sites at metal/support interface act as dynamic ligands surrounding the metal surface that confine highly active Ag nanoclusters during reactions," said Prof. FU.
This work was supported by key projects from the Ministry of Science and Technology, the National Natural Science Foundation of China, and the Carbon Neutrality Photon Science Center of the Chinese Academy of Sciences.