The group of Nano and Interfacial Catalysis is affiliated with State Key Laboratory of Catalysis of Dalian Institute of Chemical Physics, CAS. There are 18 research staffs in the Group, including 1 academician of Chinese Academy of Sciences, 4 recipients of the National Science Fund for Distinguished Young Scholars, 2 100 Talent Professors of CAS, and 7 full professors. The group and its group members have received many awards and honors during the past 20 years, including the National Natural Science Award (1st class) in 2020, the National Natural Science Award (2nd class) in 2005, Natural Science Awards of Liaoning Province (1st class) in 2009 and 2019, respectively, the National Innovation Plaque in 2017, the Ho Leung Ho Lee Foundation Award in 2012 (Prof. Xinhe Bao), the Award in Basic Science from Zhou Guang Zhao Foundation in 2015 (Prof. Xinhe Bao), the Award of Excellence in Natural Gas Conversion from the Natural Gas Conversion Board in 2016 (Prof. Xinhe Bao), the Alwin Mittasch Prize from the German Catalysis Society (GeCatS) and DECHEMA e.V. (Society for Chemical Engineering and Biotechnology) (Prof. Xinhe Bao), Tan Kah Kee Science Awards in 2018 (Prof. Xinhe Bao) and China Youth Science and Technology Award in 2019 (Prof. Xiulian Pan).
Aiming at the efficient energy conversion and optimized utilization of resources, the group of Nano and Interfacial Catalysis focuses on the development of basic concepts of catalysis, creation of highly efficient catalytic and energy conversion processes, and development of advanced in-situ or operando characterization methods and techniques. The members are carrying out researches in the fields of nano-confined catalysis, surface science and interface catalysis, catalytic conversion of C1 molecules including thermal and electro-catalysis, and thermoelectric materials and devices.
Nano-Confined Catalysis
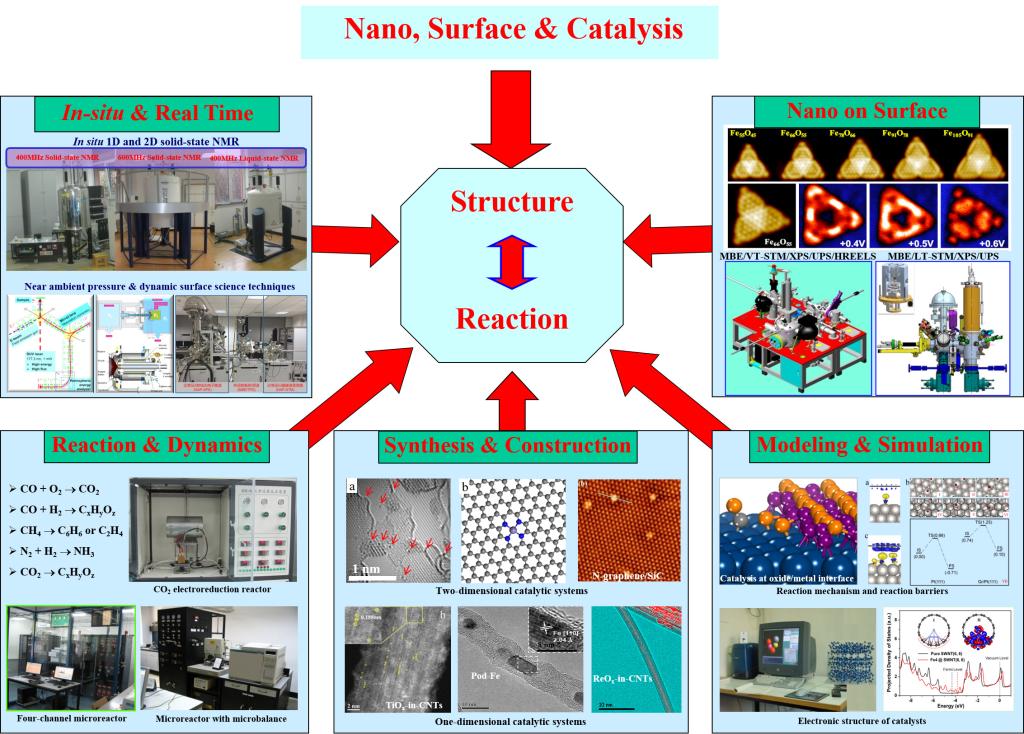
Catalysis plays an important role in the fields of energy, materials, environment and human health. It remains a challenge to reveal the catalytic reaction mechanism and rationally design catalytic active sites on the molecular and atomic scale in the field of catalysis, which relies on fundamental understanding of the reaction mechanism. With the energy conversion and resource utilization as mission, the Group is devoted to the understanding of the formation and evolution of catalytic active sites for the related processes based on the fundamental principle of nano systems, and the regulation of catalytic reaction using the concept of “Nano-Confined Catalysis”, including the confinement within the nanospace of CNTs and the interface-confinement. The “Nano-confinement” is essentially the driving force to generate and stabilize the coordinatively unsaturated active centers and to maintain its catalytic cycle. These fundamental understandings have guided successfully the design and application of nano-confined catalysts, represented by selective conversion of syngas-to-C2 oxygenates and syngas-to-light olefins, as well as selective CO oxidation. The concept of “Nano-Confined Catalysis” has become an important research topic in the field of catalysis. The Group was consequently awarded the National Natural Science Award (1st class) in 2020.
Surface Science and Interface Catalysis
Surface/interface chemistry lies at the heart of many important subjects, such as energy chemistry, catalysis and electrochemistry. It is important to understand the surface and interface effect at the atomic and molecular sale based on in-situ or operando characterizations. Guided by the concepts of dynamic chemistry and nano-confined catalysis, the Group has conducted systematic studies in the following three aspects. 1) Surface chemistry methodology: to develop new surface science instruments and establish a surface characterization platform for near ambient pressure or ambient pressure surface characterizations of gas-liquid and liquid-solid interfaces. 2) Surface and interface catalysis: to study dynamic chemistry and confined catalysis at interfaces of oxide/metal, metal/oxide, and oxide/oxide based on in-situ surface analysis. 3) Surface and interface electrochemistry: to achieve operando visualization of surface/interface processes in electrochemical model devices.
Efficient conversion and utilization of carbon-based resources and related nanomaterials
China has set the strategic target to realize "carbon neutral" in 2060, which brings new opportunities and challenges for optimal utilization of carbon resources such as coal, natural gas and biomass. The essential scientific problems lie in efficient conversion of C1 molecules such as CO and CH4. With this mission, the Group is devoted to the following studies and representative achievements: 1) Syngas conversion: A bifunctional catalyst concept, i.e. OXZEO® (Oxide-zeolite), has been developed by combining metal oxides with interface confinement and zeolites with nanospace confinement. It enables direct syngas conversion to light olefins with a selectivity reaching 80%, breaking the theoretical limit predicated by Anderson-Schultz-Flory model via the conventional Fischer-Tropsch Synthesis route. By collaborating with Professor Zhongmin Liu and his group, and Shaanxi Yanchang Petroleum Group Cooperation, we have accomplished 1000-tons annual scale pilot-plant test. 2) CH4 direct conversion: Silica lattice confined single site iron catalyst and zeolite pore confined single site molybdenum catalyst have been synthesized. We are interested in the understanding of confinement effects in the non-oxidative, direct conversion of methane to olefins, aromatics and hydrogen. 3) The team is also dedicated to development of carbon-based nanomaterials for catalysis of ammonia synthesis under mild conditions, acetylene chemistry including hydrochlorination, selective hydrogenation and carbonylation.
Thermoelectric materials and devices
Thermoelectric (TE) materials can realize the direct and reversible conversion between electric and thermal energy, showing great potentials in the fields of thermoelectric generator, solid-state refrigeration and sensors. Based on unique surface and interface modulation strategies, the Group aims to develop advanced TE materials, devices and nanoscale characterization techniques. In recent years, we have achieved several innovative progresses, which are listed as follow. We developed TMDC、CrN、GeSe and SiTe-based TE materials; Interface thermoelectrics was originally proposed to improve the TE performance of thin-film heterostructure; High-performance TE devices and systems for low-temperature applications were developed; Uncooled photothermoelectric detectors based on SrTiO3 and tellurium were developed; Scanning thermal microscopy was developed to characterize TE properties at the nanoscale.
Electrocatalytic conversion of carbon-based resources
The conversion and utilization of carbon-based resources is facing the national demands of carbon neutrality, resource diversification and sustainable environmental development. There is an urgent need to develop new processes towards the comprehensive utilization of carbon-based resources. Carbon dioxide electrolysis not only improves the utilization efficiency of carbon-based resources, but also couples with intermittent renewable energy or surplus nuclear energy to achieve large-scale energy conversion and storage. The Group designed and prepared a group of highly efficient carbon dioxide catalysts, assembled alkaline membrane and solid oxide carbon dioxide electrolyzers with industrial-scale current densities, and achieved highly efficient and stable carbon dioxide electrolysis; developed electrochemical in situ spectroscopy and high-resolution electron microscopy characterization techniques to reveal reaction mechanisms of carbon dioxide electrocatalytic reduction; explored new process for electrochemical co-conversion of carbon dioxide/carbon monoxide and carbon dioxide/methane to C2 chemicals.