Recently, the research team of Prof. Guoxiong Wang and Prof. Xinhe Bao in the State Key Laboratory of Catalysis revealed reversible exsolution/dissolution mechanism of CoFe alloy nanoparticles in Co-doped Sr2Fe1.5Mo0.5O6-δ perovskite cathode in solid oxide electrolysis cell (SOEC) for CO2 electrolysis. The results were published in Advanced Materials.
SOECs are able to convert CO2 and H2O to syngas, hydrocarbon fuel at cathode, and produce pure oxygen at anode. SOECs have the advantages of possessing solid and modular structure, fast reaction kinetics, high energy efficiency and low cost. Therefore, SOECs have promising applications in CO2 conversion and surplus renewable electricity storage.
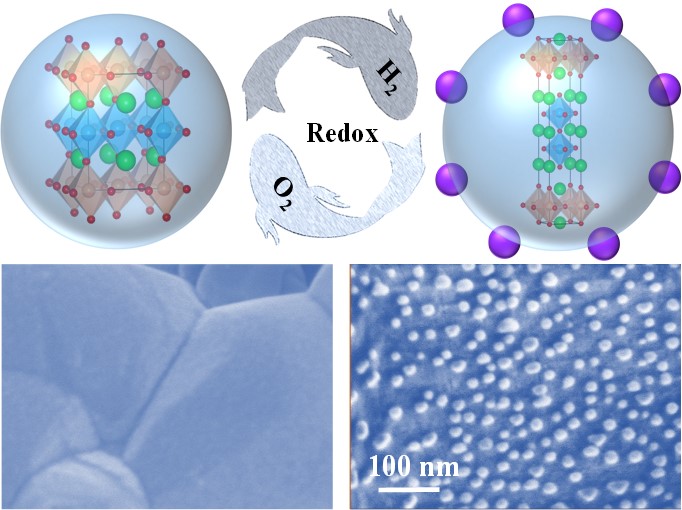
Perovskites have been extensively investigated as the most promising cathode materials for direct CO2 electrolysis in SOEC in the absence of protective gas, but many of them still suffer from insufficient CO2 electrolysis performance. In situ exsolving metal nanoparticles on the surface of perovskite have been explored as an efficient strategy to improve CO2 electrolysis performance, where abundant metal-oxide interfaces are generated for highly efficient CO2 electrolysis. Generally, reversible exsolution/dissolution of metal nanoparticles in perovskite have been proposed as vital properties for resolving the possible particle agglomeration and coke formation to enhance the stability. Until now, although some perovskites have demonstrated redox reversibility with exsolution and dissolution of metal nanoparticles in reducing and oxidizing atmosphere, fundamental understanding of these phenomena is still scarce and needs to be further investigated.
In this recently published paper, they take advantage of in situ X-ray diffraction, in situ scanning transmission electron microscopy, environmental scanning electron microscopy and density functional theory calculations to investigate the exsolution/dissolution mechanism of CoFe alloy nanoparticles in Sr2Fe1.35Mo0.45Co0.2O6-δ (SFMC) double perovskite. Under reducing atmosphere, the facile exsolution of metallic Co promotes the reduction of Fe cation to generate CoFe alloy nanoparticles in SFMC, accompanying with the structure transformation from double perovskite to layered perovskite. Under oxidizing atmosphere, the spherical CoFe alloy nanoparticles are firstly oxidized to flat CoFeOx nanosheet, and then dissolved into the bulk accompanying with structure transformation from layered perovskite back to double perovskite. After reduction, metal-oxide interfaces between the exsolved CoFe nanoparticles and SFMC substrate with oxygen vacancies are constructed, which shows enhanced CO2RR performance and high stability. The CO2 electrolysis performance can be retrieved after 12 redox cycles due to the renewability of CoFe nanoparticles.
This work was supported by the National Natural Science Foundation of China the National Key R&D Program of China and DICP. (Text and Image by Houfu Lv and Le Lin).