Recently, the research team of Prof. Guoxiong Wang and Prof. Xinhe Bao in the State Key Laboratory of Catalysis was invited to publish a progress report entitled “High-Temperature CO2 Electrolysis in Solid Oxide Electrolysis Cells: Developments, Challenges, and Prospects” in Advanced Materials (DOI: 10.1002/adma.201902033).
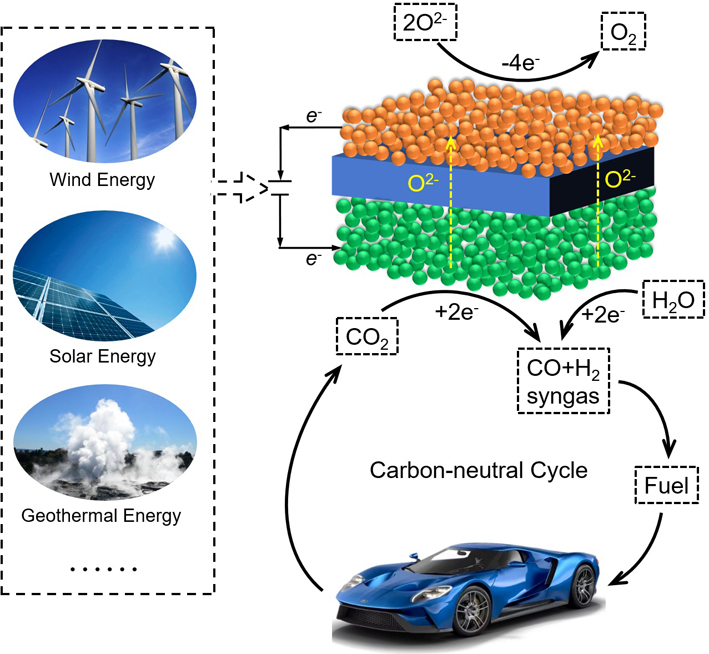
Solid oxide electrolysis cells (SOECs) are able to convert CO2 and H2O to syngas, hydrocarbon fuel at cathode, and produce pure oxygen at anode. SOECs have the advantages of possessing solid and modular structure, fast reaction kinetics, high energy efficiency and low cost. Therefore, SOECs have promising applications in CO2 conversion and surplus renewable electricity storage. Here, this progress report summarizes the development history of SOEC, reaction mechanism of CO2 electroreduction, electrolyte materials and electrode materials, and introduces the effect of SOEC microstructure on the electrochemical performance, and highlights the SOEC degradation,the advances of fuel-assisted SOEC and high-pressurized SOEC, and finally makes a prospect for the future research such as utilizing density in situ physiochemical characterizations to monitor the elementary processes of the electrode reactions and incorporating the alkane conversion at anode of the SOEC to efficiently produce fuel and chemicals.
The research team of Prof. Guoxiong Wang and Prof. Xinhe Bao has been dedicated to high-temperature CO2 electrolysis in recent years, and has constructed a series of metal-oxide interfaces by infiltration and in situ exsolution, which increases the amount of surface oxygen vacancies, and promotes the CO2 activation at cathode and oxygen evolution reaction at anode, leading to the enhanced electrochemical performance. The results of electrochemical impedance spectroscopy, in situ electrochemical X-ray photoelectron spectroscopy and density functional theory calculations reveal that the metal-oxide interface at cathode facilitates the dissociative adsorption of CO2, while the metal-oxide interface at anode triggers the oxygen spillover form oxide to metal surface. And the research work have been published in Angew. Chem. Int. Ed. 2019; Nano Energy 2018; Energy Storage Mater. 2018; J. Mater. Chem. A 2018; J. Mater. Chem. A 2019 and so on.
This work was supported by the National Science Foundation of China (Grants 21573222, 91545202, and 21703237), the National Key R&D Program of China (Grant 2017YFA0700102), and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant XDB17020200). This work is dedicated to the 70th anniversary of Dalian Institute of Chemical Physics, Chinese Academy of Sciences. (Text/Image by Yuefeng Song)