Identification of Active Sites over MCM-49 for Direct Syngas Conversion to Gasoline
Recently, a research team led by Prof. Xiulian Pan and Prof. Xinhe Bao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has made new progress in the direct syngas conversion, focusing on the roles of different pore systems of MCM-49 during the oxide-zeolite composites for syngas-to gasoline (OXZEO-TG) process.
Syngas is a key platform for the utilization of carbon resources including coal, natural gas, and biomass. The conversion of syngas to gasoline with high selectivity has been widely concerned in both industry and academia. OXZEO process, as an alternative process for syngas conversion, has made great progress in the direct conversion of syngas to olefins, aromatics and gasoline since 2016. Our previous report shows that ZnCrOx-MCM-22 could efficiently convert synthetic gas into gasoline components rich in iso-paraffins (Appl. Catal. B, 2022, 316, 121628), but the role of the different pore systems of MCM-22 (MWW-type zeolite) is still unclear.
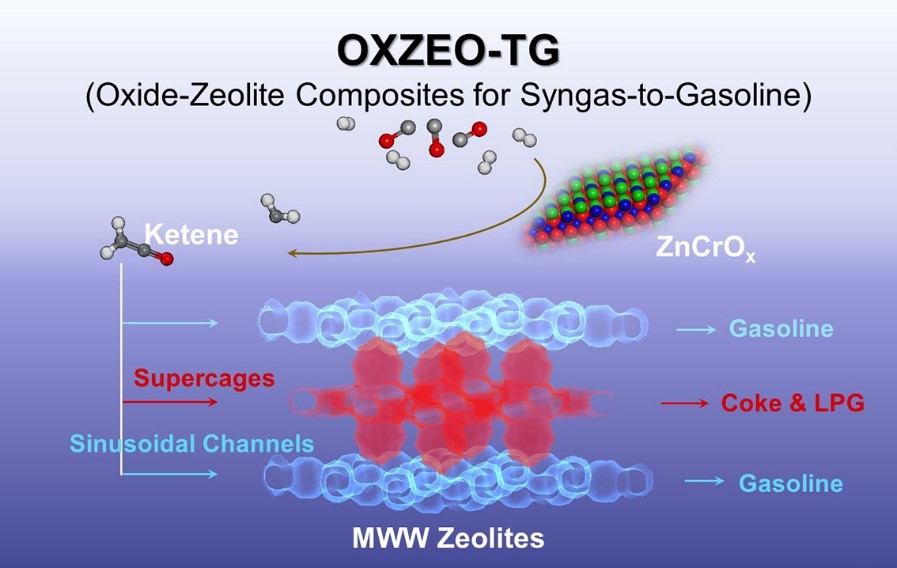
Herein, we show that the accessibility of the acid sites in different pores of MCM-49 is tuned by sulfuric acid during crystallization, as revealed by positron annihilation lifetime spectroscopy (PALS) and infrared (IR) spectroscopy. This allows the identification of the active sites for selective formation of gasoline in direct syngas conversion over the ZnCrOx-HMCM-49 (OXZEO) catalyst. It reveals that the acid sites in the sinusoidal channels are active for gasoline formation, whereas the acid sites in the supercages favor the production of light paraffins and are also prone to deactivation.
This work was published in ACS Catalysis with the title “Identification of Active Sites over MCM-49 for Direct Syngas Conversion to Gasoline”. The work was supported by the National Natural Science Foundation of China, the Natural Science Foundation of Liaoning Province, Dalian High-level Talent Innovation Program and the Innovation Research Fund of Dalian Institute of Chemical Physics. (Text/Picture by Yilun Ding)
Article link: https://doi.org/10.1021/acscatal.3c02003