Iron-single sites confined by graphene lattice for ammonia synthesis under mild conditions
A research team led by Prof. PAN Xiulian and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) reported iron-single sites confined by graphene lattice for ammonia synthesis under mild conditions.
Ammonia as a primary feedstock for the synthesis of fertilizer, medicine, polyester, etc., is one of the mostly manufactured chemicals in the world. The Haber-Bosch process, developed in the early 20th century, is still the primary industrial ammonia synthesis technology. The inert N≡N bond is known to be highly stable with a bond energy as high as 941 kJ mol-1. Therefore, the Haber-Bosch process requires extreme conditions, i.e. a pressure as high as 200-400 bar in a temperature range of 400-600 ℃. It was reported that ammonia synthesis industry consumes approximately 2% of the global energy consumption and gives 1.6% of global CO2 emissions. Since the reaction is thermodynamically more favored at a lower temperature, it is desirable to develop a highly active catalyst to allow the reaction at a lower temperature and pressure. This will save some of the energy cost although it does not affect the energy cost of hydrogen production. However, no ammonia formation was detected below 150 ℃ over a commercial Fe catalyst. In comparison, nitrogenase can enable N2 fixation under ambient conditions. Its active site was reported to be a [Mo:7Fe:9S:C]:homocitrate cluster (FeMo-cofactor), which contained isolated Fe atoms being coordinated with S or C atoms. Additionally, electrons are transferred in nitrogenase from the Fe protein to the MoFe protein concomitantly with ATP hydrolysis. Therefore, development of heterogeneous catalysts by mimicking the structure of nitrogenase and its catalytic mechanism has become the efforts of worldwide researchers for decades.
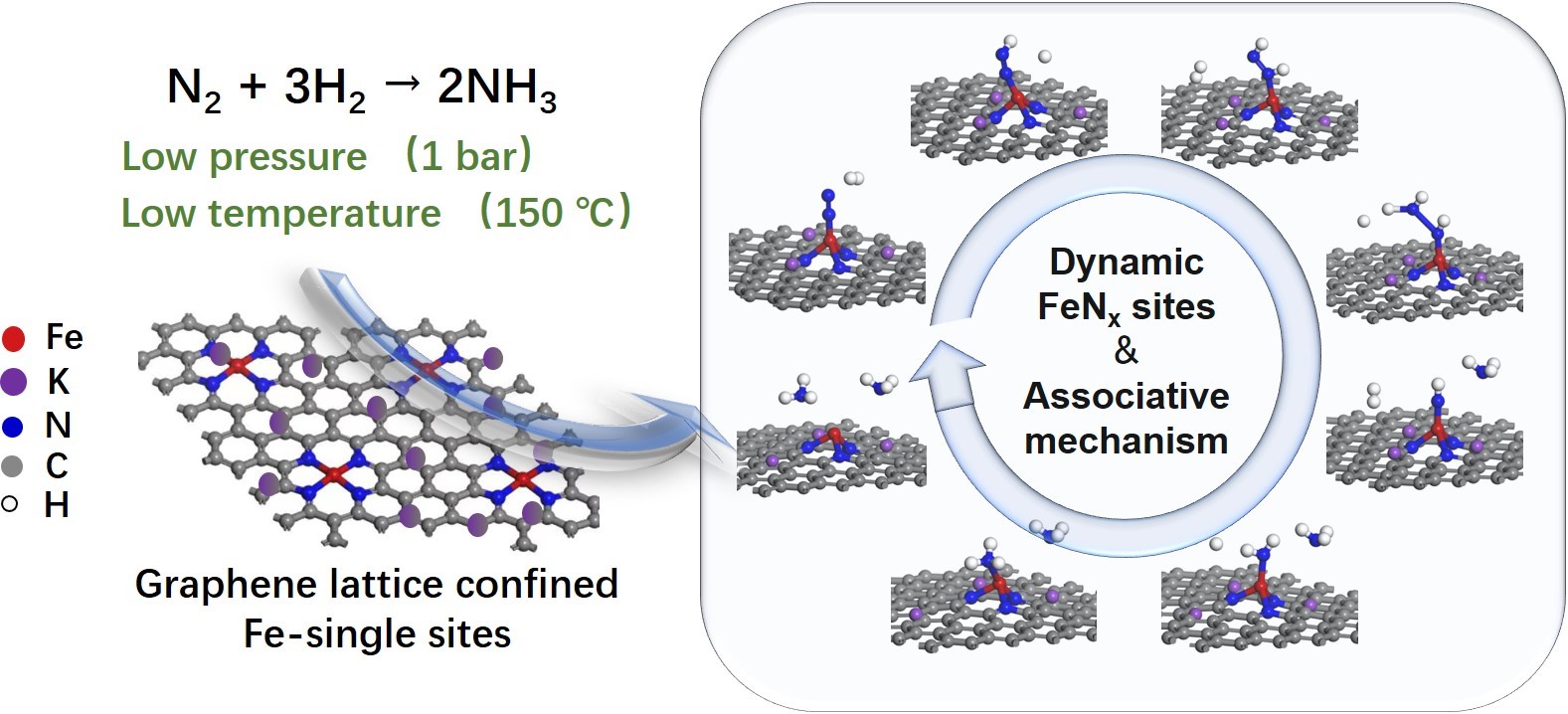
In this work, the researchers reported that an iron-single-site catalyst confined within the graphene lattice derived from ball-milling of iron phthalocyanine with graphene and promoted by alkali metal enables ammonia synthesis below 200 ℃ and atmospheric pressure. The catalyst exhibits an activity of 1.1 μmolNH3 gc-1 h-1 at 150 ℃ and 10.3 μmolNH3 gc-1 h-1 at 190 ℃ whereas no activity is detected over a physical mixture of iron phthalocyanine and graphene. By the characterization with X-ray absorption fine structure, the researchers revealed that iron-single sites are stabilized as FeN4 sites within the graphene lattice and evolve to FeN3 during reaction. The researchers found that N2 transformation to NH3 follows an associative mechanism and the isotope experiments indicate that N atoms of FeN3 participate in the reaction, which in turn are replenished from N2. It resembles that of the mimetic-enzyme structure and its reaction mechanism.
This work was supported by the Ministry of Science and Technology of China. (Text by CHEN Ziquan)