Recently, on the basis of their previous studies towards two-dimensional catalytic materials and nano confined catalysis, Prof. Dehui Deng and Prof. Xinhe Bao etc. from SKLC, DICP, found that single iron atoms confined in graphene (FeN4/GN) can be used as an efficient non-precious catalyst to convert methane to high value-added C1 oxygenated products at room temperature. This work has recently been published as a research article in Chem (Chem, 2018, 4, 1902-1910. DOI: 10.1016/j.chempr.2018.05.006).
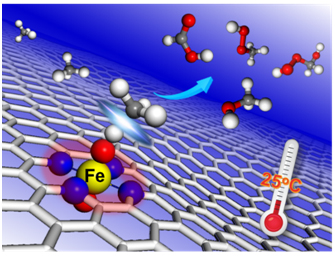
Methane from natural gas, shale gas and methane hydrate is a most promising feedstock because of its natural abundance and relatively inexpensive price. The selective activation and orientable conversion of methane is considered as the "holy grail" in catalysis. Owing to the highly stable C-H bond (ΔHC–H = 104 kcal mol-1), methane conversion usually requires high temperatures (600-1100 oC) to overcome the high reaction barrier. Despite many efforts to decreasing the reaction temperature, it remains a great challenge to promote methane conversion under mild conditions without the aid of plasma, electrical, or photo, especially at room temperature. Inspired by their previous studies on the two dimensional nanomaterials for C-H activation under mild conditions, such as oxidation of benzene to phenol (Sci. Adv. 2015, 1, e1500462) and methanol into ethylene glycol (Nat. Commun. 2018, 9, 1181), they found that graphene-confined single Fe atoms, screened out from a series of 3d transition metals (Mn, Fe, Co, Ni, Cu), can be used as an efficient non-precious catalyst to directly convert methane to C1 oxygenated products with H2O2 as oxidant at room temperature. In collaborations with Prof. Xiuwen Han and Prof. Haiyang Li et al., they found that the methane conversion proceeds on the O-FeN4-O active site along a radical pathway to produce CH3OH and CH3OOH firstly, and then the generated CH3OH can be further catalyzed to HOCH2OOH and HCOOH by combining with the operando time-of-flight mass spectrometry, 13C nuclear magnetic resonance and density functional theory calculations. And the moderate formation energy of O-FeN4-O results in the unique activity for methane conversion at room temperature compared with other graphene-confined transition metals. These findings in the present work pave a new route to understand and design highly efficient non-precious heterogeneous catalysts for methane conversion at room temperature. This work has received extensive attention from home and abroad. The Royal Society of Chemistry website has highlighted the work with the topic of “Catalyst Converts Methane to Methanol at Room Temperature” on the Chemistry World. In addition, Prof. Ye Wang from Xiamen University introduced the work in the Joule with the title "Room-Temperature Conversion of Methane Becomes True". He evaluated the work as a significant breakthrough in methane chemistry. He also said “The work not only provides an important clue for the design of highly active methane-conversion catalysts working under mild conditions but also will definitely encourage more research in this area”.
These works are supported by Ministry of Science and Technology of China, National Natural Science Foundation of China, Key Research Program of Frontier Sciences of the Chinese Academy of Sciences, Strategic Priority Research Program of the Chinese Academy of Sciences, National Postdoctoral Program for Innovative Talents, and Collaborative Innovation Center of Chemistry for Energy Materials (2011. iChEM). (by Xiaoju Cui and Hehua Gao)