Recently, Prof. WANG Guoxiong and Prof. BAO Xinhe from the State Key Laboratory of Catalysis made new progress in the research of electrocatalytic carbon dioxide reduction reaction (CO2RR). This team achieved high activity of CH4 production in CO2RR on a non-copper-based catalyst, and provided a new strategy for the electrocatalytic reduction of carbon dioxide to hydrocarbons.
CO2RR uses renewable electricity to convert carbon dioxide and water into fuels and chemicals, which is considered as an effective way to simultaneously realize carbon recycling and renewable energy storage. The electrocatalytic conversion of carbon dioxide into hydrocarbons involves a multi-electron reduction reaction process, with enormous scientific problems such as complex conversion pathways and difficulty in selectivity control. The current research is mainly focused on copper-based catalysts, however, the active sites of Cu(I) species in Cu based catalysts are prone to be reduced and become inactivated under CO2RR conditions. There are only a few reports on non-copper catalysts, and the development of non-copper catalysts for electrocatalytic reduction of carbon dioxide to hydrocarbons is worthy to be explored.
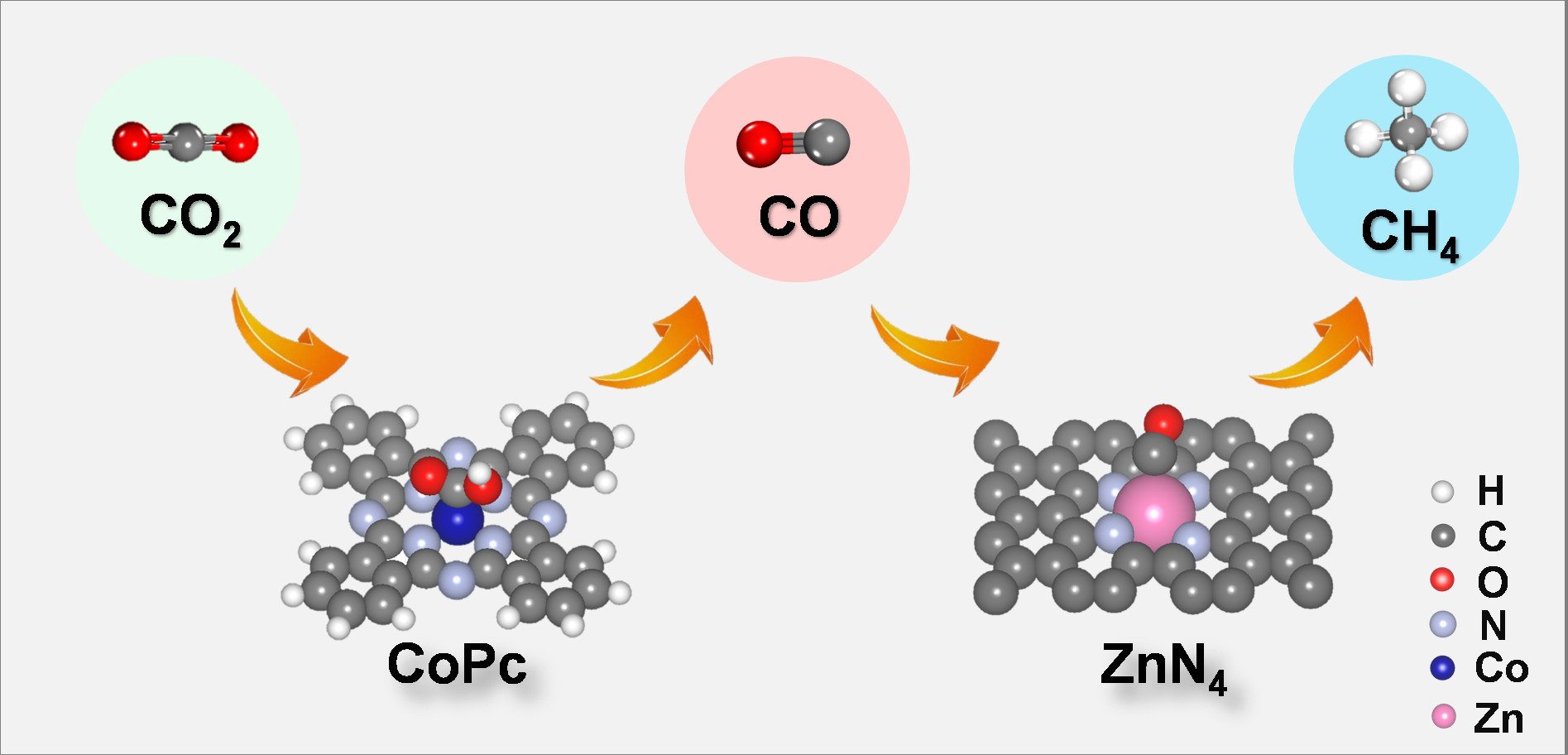
In this work, the researchers reported a cobalt phthalocyanine (CoPc) and zinc-nitrogen-carbon (Zn-N-C) tandem catalyst, which can electrochemically convert carbon dioxide into methane effectively. Compared with CoPc or Zn-N-C alone, the methane/carbon monoxide rate ratio of this tandem catalyst is increased by more than 100 times. Density functional theory calculations and comparative experimental results show that carbon dioxide is first reduced to carbon monoxide on the CoPc, and then the carbon monoxide diffuses onto the Zn-N-C and is further converted into methane. This tandem catalytic strategy converts carbon dioxide into methane and decomposes the process into tandem electrocatalytic reactions at two active sites. In this tandem catalytic system, CoPc provides carbon monoxide to retain the adsorbed hydrogen on the adjacent-nitrogen in the Zn-N site, thereby increasing the rate of methane production.
Related work was published in Angew. Chem. Int. Ed.. This work was funded by the National Natural Science Foundation of China, the National Key Research and Development Program, and the Youth Innovation Promotion Association of the Chinese Academy of Sciences. (Text/Picture LIN Long and LIU Tianfu)