Electrocatalytic CO2 reduction reaction (CO2RR), using clean electricity to convert CO2 and water into chemicals and fuels, is considered as an effective way to simultaneously close the carbon cycle and store renewable energy.
The products of CO2RR are very complex. Compared with C1 products, it’s more difficult to generate C2+ products due to the multiple proton-electron transfer, the complex intermediates and the sluggish C-C coupling step during CO2RR to C2+ products, leading to low selectivity and production rate for C2+ formation.
Recently, a research team led by Prof. WANG Guoxiong, Prof. GAO Dunfeng and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) designed a Cu-CuI composite catalyst, achieving a highly efficient production of multicarbon (C2+) chemicals from CO2RR.
This study was published in Angewandte Chemie International Edition on April 10.
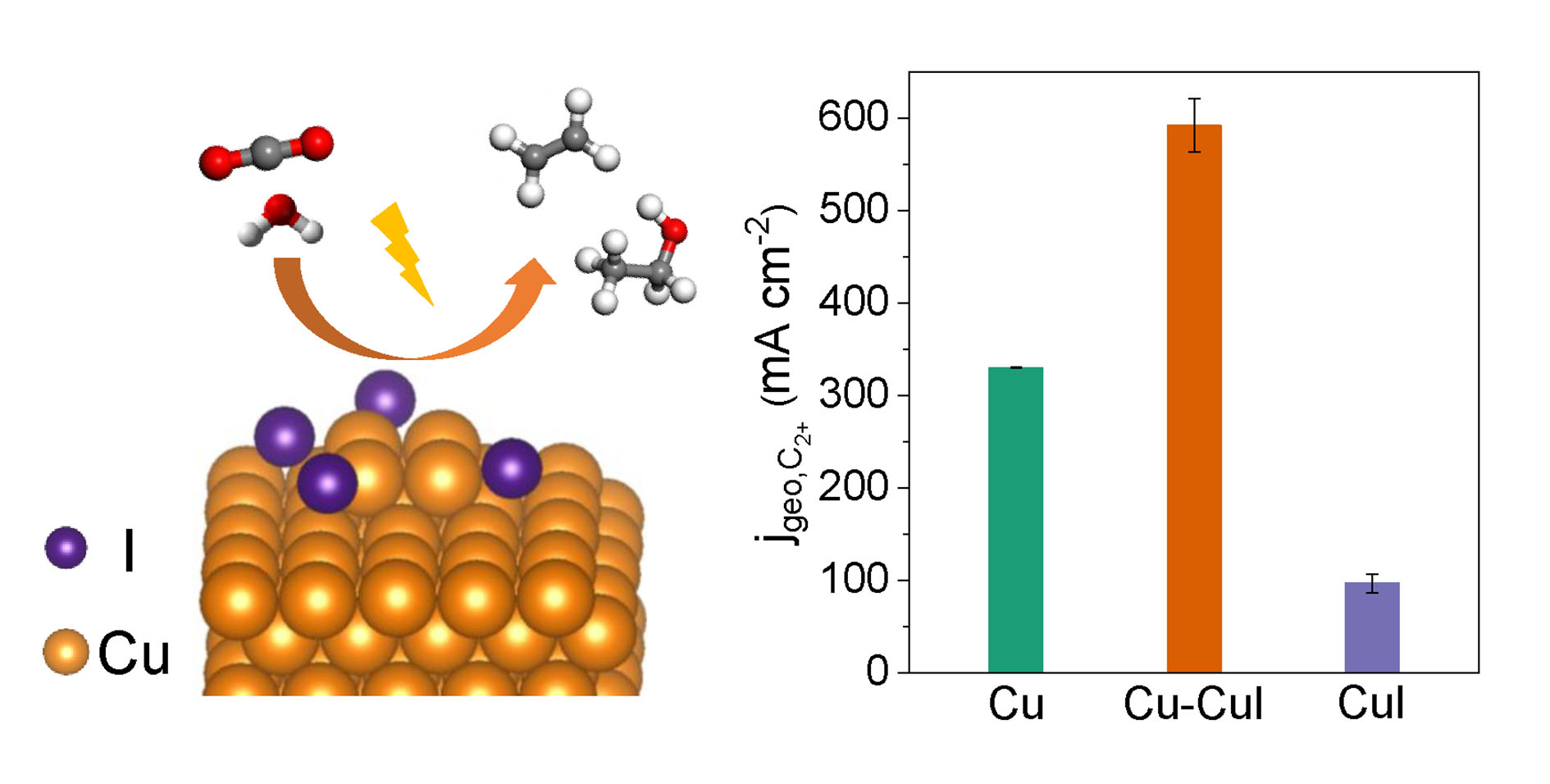
A Cu-CuI composite catalyst achieves highly efficient production of C2+ chemicals from electrocatalytic CO2 reduction.
The researchers designed a Cu-CuI composite catalyst with abundant Cu0/Cu+ interfaces by physically mixing Cu nanoparticles and CuI powders. The composite catalyst could achieve a maximum C2+ Faradaic efficiency of 71% and a remarkable C2+ partial current density of 591 mA cm−2 at −1.0 V vs. reversible hydrogen electrode (RHE) in a flow cell, which was higher than Cu and CuI counterparts. During 85-hour stability test at −0.8 V vs. RHE, the geometric current density maintained at ~550 mA cm−2 and the Faradaic efficiencies of C2+ and ethylene declined slightly.
Structural characterizations indicated that the Cu-CuI composite catalyst underwent significant reconstruction under CO2RR conditions, which was induced by alkaline electrolyte and applied potential. The high-rate C2+ production over Cu-CuI was ascribed to the presence of residual Cu+ and adsorbed iodine species which improved CO adsorption and facilitate C-C coupling.
“This work presents a new strategy for designing efficient catalysts towards high-rate CO2RR to C2+ products,” said Prof. WANG.
The above work was supported by the National Natural Science Foundation of China, the National Key Research and Development Program, and the Youth Innovation Promotion Association of CAS. (Text by LI Hefei and LIU Tianfu)