Recently, the research team led by Professor Xiulian Pan and Associate Professor Guangzong Fang from Group 522, Nano and Interfacial Catalysis Research Center, has achieved new progress in the field of vinyl chloride production via acetylene hydrochlorination. They designed and fabricated a boron nitride-confined nanographene (BNC) metal-free catalyst, which realizes efficient acetylene conversion by constructing abundant interfacial B-N-C active sites.
As one of the most important engineering plastics globally, polyvinyl chloride (PVC) is widely used in construction, packaging, electronics, and other fields. Currently, 35% of global PVC production relies on the acetylene hydrochlorination reaction, which predominantly uses mercury-based catalysts. Driven by global environmental initiatives and the requirements of the Minamata Convention, the development of efficient and non-toxic alternative catalysts has become an urgent demand for industrial development. However, the existing noble metal catalysts under development suffer from high costs and easy deactivation, while non-noble metal catalysts face bottlenecks of insufficient activity and stability, making them difficult to meet industrial application requirements.
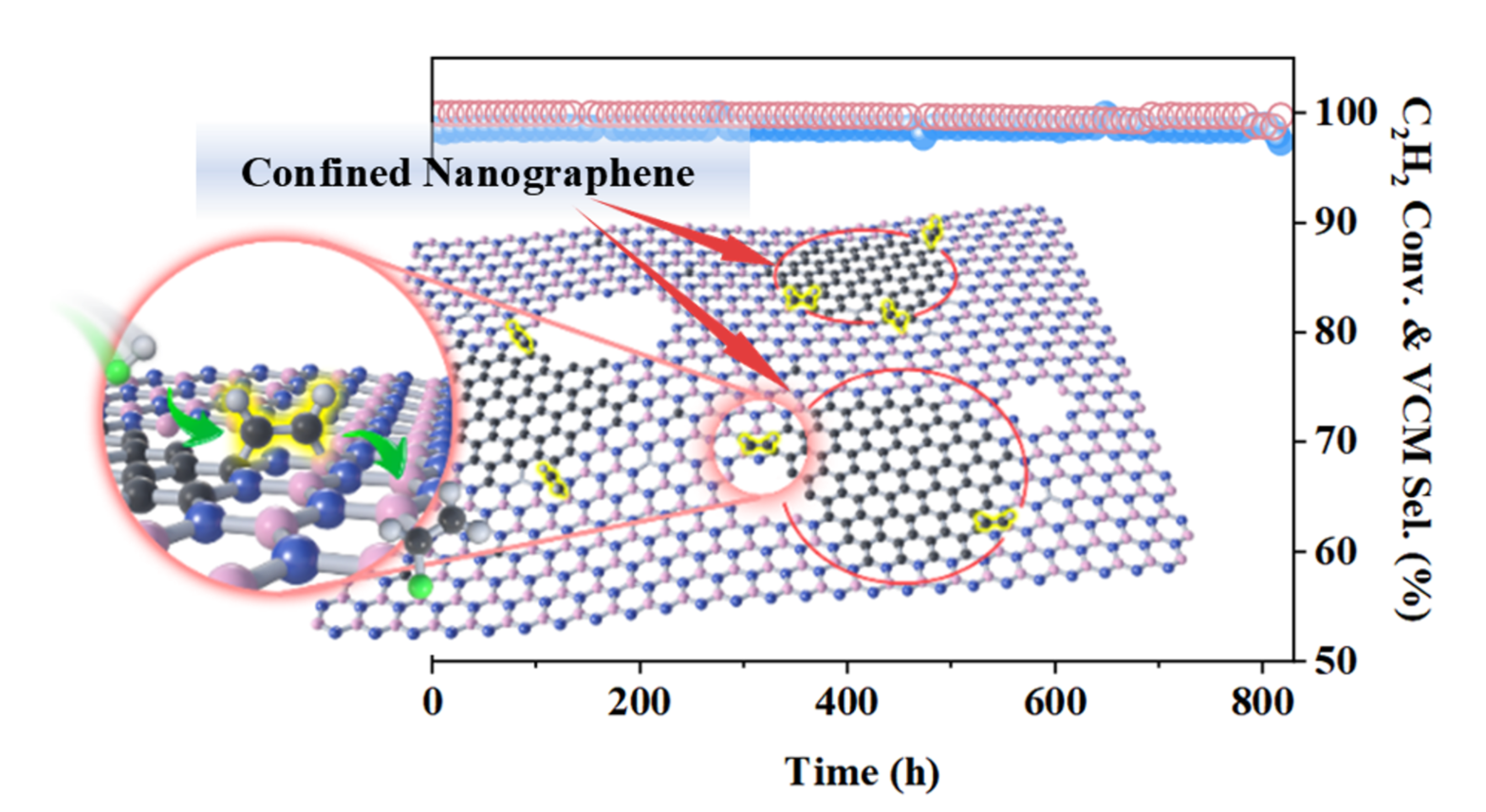
Professor Xiulian Pan’s team has long been committed to research in the field of carbon-based energy catalytic conversion. Building on their previous work on metal-free catalyzed acetylene hydrochlorination for vinyl chloride production (Nature Communications, 2014; ACS Catalysis, 2017; Applied Catalysis B: Environmental, 2017), the team introduced nanographene into the boron nitride framework to give a unique two-dimensional confined catalyst with abundant interfacial B-N-C active sites. The unique electronic structure of these sites can effectively promote the adsorption and polarization of acetylene molecules, thereby enhancing catalytic performance.
Experimental results show that under reaction conditions of 260 °C and an acetylene gas hourly space velocity (GHSV) of 45 h⁻¹, the catalyst maintains acetylene conversion and vinyl chloride selectivity both approaching 99% after 800 hours of continuous operation. Even when the GHSV is increased to 650 h⁻¹, the acetylene conversion remains at 81%, and the vinyl chloride time-space yield (TSY) reaches 4.53 g·gcat⁻¹·h⁻¹. This metal-free catalyst features a simple preparation process and low cost, providing a feasible pathway for the development of mercury-free alternative catalysts and holding great promise for promoting the green and sustainable development of the vinyl chloride industry.
Related research results, entitled “Boron Nitride-Confined Nanographene as a Metal-Free Catalyst for Acetylene Hydrochlorination,” have recently been published in the Journal of the American Chemical Society (JACS). The first authors of this work are Ph.D. student Xiaoqiang Guo and Yihan Ye, as well as Associate Professor Guangzong Fang. This research was supported by the National Key R&D Program of China, the National Natural Science Foundation of China (NSFC), and Leading Special Project Class A of the Chinese Academy of Sciences “Clean Combustion and Low-Carbon Utilization of Coal.” (Text/Photos: Guangzong Fang, Xiaoqiang Guo)
Article Link: https://pubs.acs.org/doi/10.1021/jacs.5c13270