Methane, the primary component of natural gas, is a low-cost feedstock and a fundamental building block in C1 chemistry. Efficiently upgrading methane into value-added C2+ hydrocarbons holds great promise for energy utilization and sustainable chemical production. However, conventional oxidative coupling and non-oxidative coupling routes suffer from severe over-oxidation and carbon deposition, respectively, which limit their further application. Developing a methane conversion pathway with high atom economy and robust stability is therefore of both scientific and industrial significance. In addition, the anodic reaction mechanism of methane in solid oxide electrolysis cells (SOECs) remains poorly understood, hindering the rational design of efficient methane-conversion anodes.
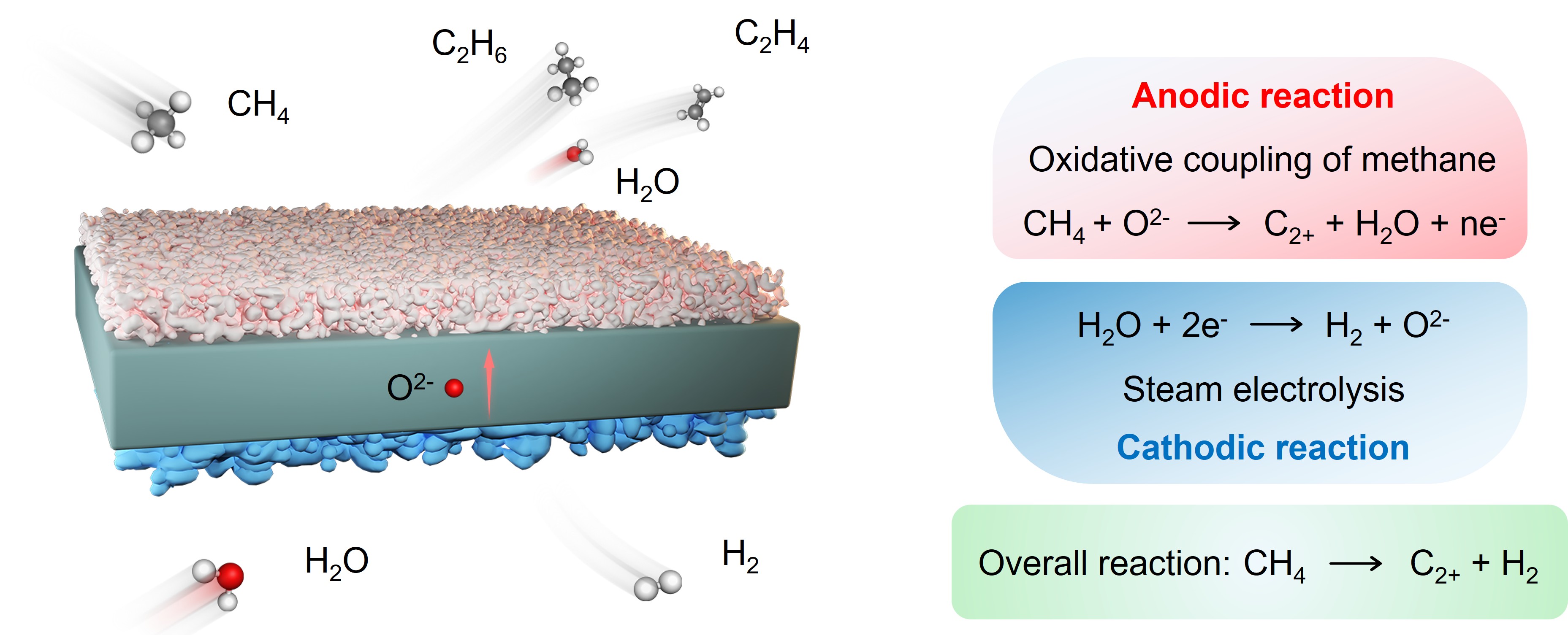
Steam–methane co-electrolysis for simultaneous C2+ hydrocarbons and hydrogen co-production (Image by GUO yige)
In a study published in Angewandte Chemie International Edition, Assoc. Prof. SONG Yuefeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. WANG Guoxiong from Fudan University, reported a novel steam–methane co-electrolysis system that simultaneously generates C2+ hydrocarbons at the anode and hydrogen at the cathode. The researchers not only realized efficient co-production but also provided mechanistic insights into anodic oxidative coupling of methane (OCM).
The co-electrolysis system demonstrated stable operation for over 90 hours at a current density of 127 mA cm⁻², achieving more than 75% selectivity toward C2+ hydrocarbons, including ~40% selectivity for ethylene, without any observable carbon deposition.
To unravel the underlying mechanism, the researchers employed in situ Raman spectroscopy and near-ambient pressure X-ray photoelectron spectroscopy (NAP-XPS). These analyses revealed that oxygen ions (O2-) driven across the electrolyte migrate onto the Ag anode surface under polarization, where they form highly reactive electrochemically spillover oxygen (ESO). Methane is then activated by ESO to produce methyl radicals (∙CH3). Density functional theory (DFT) calculations further confirmed that ESO coverage on Ag significantly enhances methane adsorption and lowers the C–H bond cleavage barrier. Synchrotron radiation photoionization mass spectrometry (SR-PIMS) provided direct experimental evidence of gas-phase ∙CH3 radicals, showing that their concentration increases with applied voltage. Together, these findings establish a “surface activation–gas-phase coupling” mechanism for anodic OCM, where surface-generated ∙CH3 intermediates desorb and couple in the gas phase to form C2+ hydrocarbons. This mechanistic framework not only clarifies the anodic methane conversion pathway in SOECs but also offers valuable guidance for the rational design of catalysts and optimization of electrochemical reactor engineering.
“Our study establishes steam-methane co-electrolysis as a new strategy for the simultaneous production of C2+ hydrocarbons and hydrogen, while also providing fundamental mechanistic insights into the electrochemical methane coupling reaction.” said Dr. Song.
Key words: Solid oxide electrolysis cell, Oxidative coupling of methane, Electrochemically spillover oxygen
Article Link: https://doi.org/10.1002/ange.202512935