Study Revealing the Chemistry of Ketene Transformation to Gasoline Catalyzed by H‑SAPO-11
Recently, a study led jointly by Prof. PAN Xiulian, Prof. BAO Xinhe and Prof. HOU Guangjin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) revealed the reaction mechanism of ketene transformation to gasoline on the H-SAPO-11. It was published in the Journal of the American Chemical Society recently.
In 2016, the team proposed a new catalyst concept based on metal oxide-zeolite bifunctional catalysts (OXZEO®) and ketene intermediate was successfully detected by highly sensitive synchrotronbased vacuum ultraviolet photoionization mass spectrometry during syngas conversion. Further studies demonstrated that ketene can be converted to mixed light olefins over SAPO-34 and transformed to ethene over the eight-membered ring (MR) side pocket acid sites of mordenite. Although ketene has also been identified as an important intermediate in other zeolite-catalyzed C1 chemistry, including methanol to hydrocarbons (MTH), dimethyl ether (DME) carbonylation to methyl acetate and CO2 hydrogenation to hydrocarbons, its transformation mechanism is not well understood.
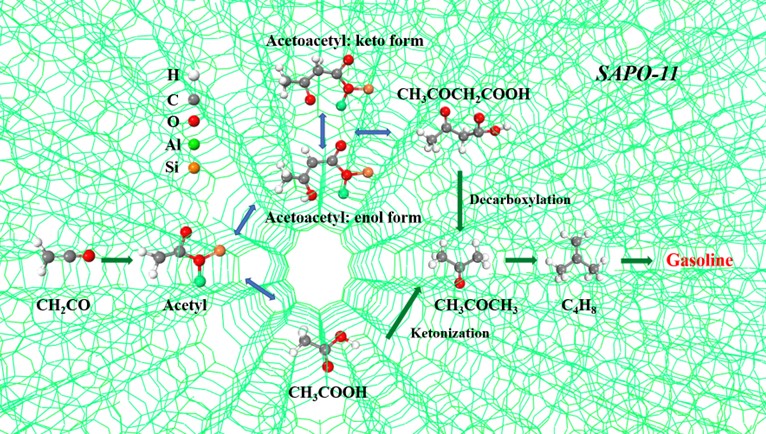
Chemistry of ketene transformation to gasoline catalyzed by H‑SAPO-11(Image by ZHANG Yang and GAO Pan)
In this study, the researchers studied ketene conversion over H-SAPO-11 employing kinetic analyses, in situ infrared spectroscopy, and solid-state nuclear magnetic resonance (ssNMR) spectroscopy. Ketene transforms to butene on the acid sites via either acetyl species following an acetic acid ketonization pathway or acetoacetyl species with keto-enol tautomerism following an acetoacetic acid decarboxylation pathway in the presence of water. These findings reveal experimentally for the first time on the reaction network of catalytic ketene conversion over zeotypes and will benefit further understanding of C1 chemistry.
This work was supported by the Ministry of Science and Technology of China, the National Natural Science Foundation of China, the Liaoning Revitalization Talents Program, the China National Postdoctoral International Exchange Program, the China National Postdoctoral Program for Innovative Talents, and the China Postdoctoral Science Foundation. (Text by ZHANG Yang and GAO Pan)