Solid oxide electrolysis cells (SOEC) provide a practical solution for direct conversion of CO2 to chemicals, however, an in-depth mechanistic understanding of the dynamic reconstruction of active sites for perovskite cathodes during CO2 electrolysis remains a great challenge.
Recently, a research team led by Prof. BAO Xinhe, Prof. WANG Guoxiong and Dr. LV Houfu from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has made new progress in the research of high-temperature CO2 electrolysis in SOEC. The iridium-doped Sr2Fe1.45Ir0.05Mo0.5O6-δ (SFIrM) perovskite displays a dynamic electrochemical reconstruction feature during CO2 electrolysis with abundant exsolution of highly dispersed IrFe alloy nanoparticles (NPs) on the SFIrM surface, which is well investigated using in situ electrochemical X-ray diffraction (XRD), near-ambient pressure X-ray photoelectron spectroscopy (NAP-XPS), and X-ray absorption spectroscopy (XAS).
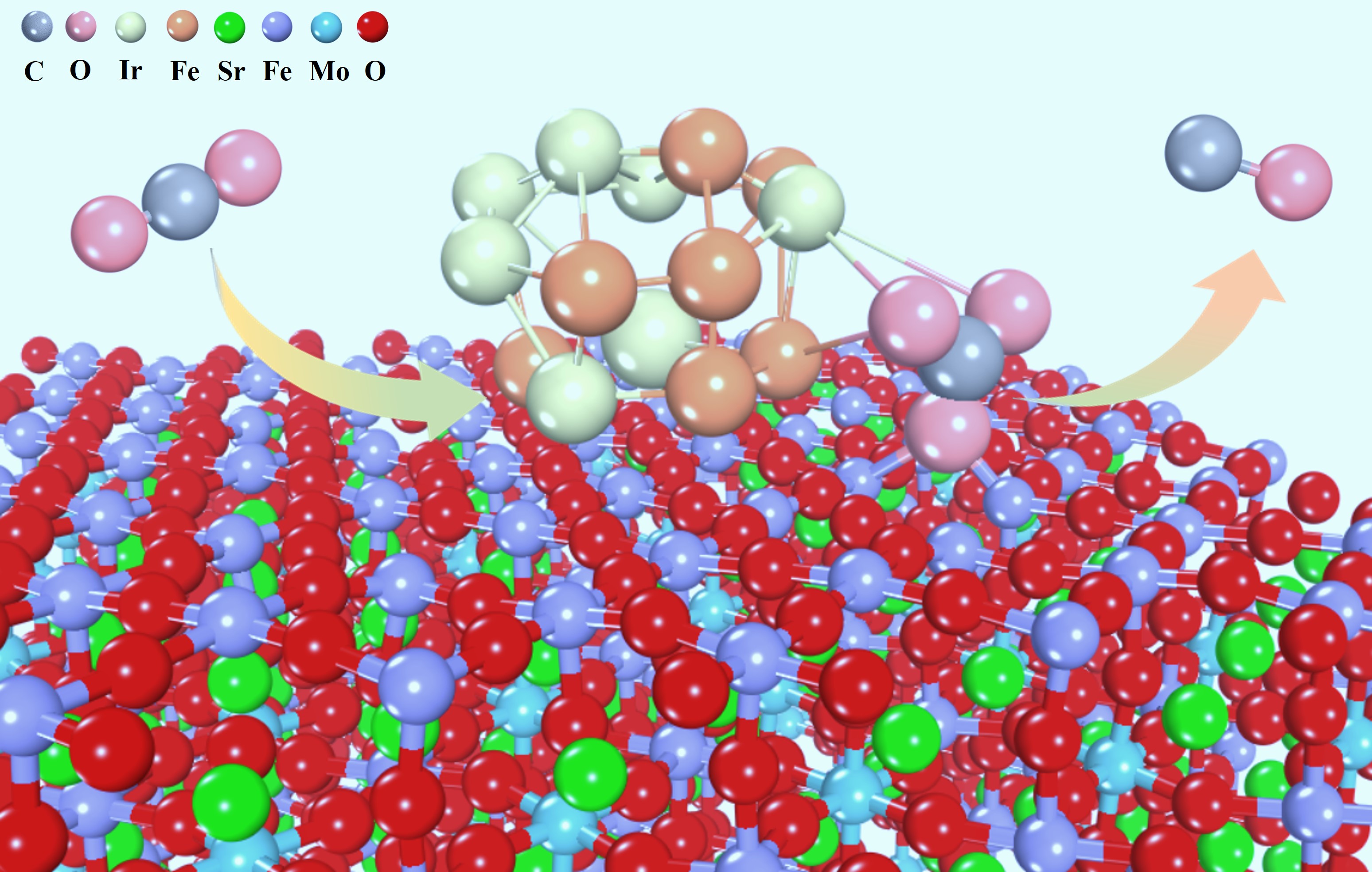
In situ electrochemical reconstruction of Sr2Fe1.45Ir0.05Mo0.5O6-δ perovskite cathode contributes to efficient carbon dioxide electrolysis in solid oxide electrolysis cell (Image by Shen Yuxiang and Liu Tianfu)
During the electrochemical reconstruction and activation process under a constant voltage mode at 1.0 V for ~4 000 s, the current density gradually increased and finally approached a steady state. The exsolved IrFe alloy NPs display high dispersion with an average diameter of ~1.0 nm and a density above 80 000 μm-2 on the SFIrM surface. Moreover, the activation time was reduced rapidly with increasing applied voltage (constant voltage: 1.2V, activation time: within 250 s), with similar particle size and density of the exsolved metal NPs during CO2 electrolysis.
In addition, in situ NAP-XPS measurements were employed to monitor their catalytic process during CO2 electrolysis to reveal the intrinsic reaction mechanism. Upon application of electrochemical polarization, a broader peak appeared at ca. 290 eV in the C 1s spectra, which could be attributed to carbonate species and was most probably decisive for CO2 electrolysis. The peak area of carbonate species on the Sr2Fe1.5Mo0.5O6-δ (SFM) cathode was weaker than that on the SFIrM cathode. The enhanced carbonate intermediate species signal confirmed that the in situ reconstructed IrFe@SFIrM interfaces facilitated CO2 adsorption and activation. IrFe@SFIrM interfaces were thus proposed as the catalytically active sites that devoted to a higher CO2 electrolysis performance than SFM.
The initial exsolved IrFe alloy NPs could be re-dispersed sufficiently into smaller nanoclusters via a brief oxidation treatment in air. IrFe alloy NPs were regenerated when the applied voltage was switched on again, and the appearance of the intermediate carbonate peak could be further obtained. The oxidative re-dispersion strategy could efficiently improve the stability by delaying particle aggregation.
“This work provides a surface reconstruction process and reactive mechanism investigation of the cathode, which may provide an in-depth understanding of CO2 electrolysis in SOEC,” said Prof. WANG.
The above work was supported by the National Key Research and Development Program of China and the National Natural Science Foundation of China. (Text/Picture Shen Yuxiang/Liu Tianfu).