Electrochemical NO3− reduction reaction (NO3−RR) represents a sustainable route for achieving carbon-free NH3 production that can address water pollution and balance the nitrogen cycle. As NO3−RR to NH3 production is a complex nine-proton and eight-electron transfer process that involves multiple reaction pathways and intermediates, developing catalysts with high activity, selectivity and stability is necessary for efficient NH3 production and NO3− removal.
Cu-based catalysts are considered as one of the most promising catalysts available for NO3−RR to NH3, owing to the similarity between energy levels of d-orbital on Cu and LUMO π* of NO3−. However, Cu catalysts only allows NH3 formation at comparatively high overpotentials overlapping with hydrogen evolution reaction (HER), which not only requires considerable energy input, but also hinders the increase of current density corresponding to NH3 production.
Recently, a research team led by Prof. WANG Guoxiong and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has made new progress in the research of electrochemical NO3−RR to produce NH3. A high-performance Cu nanosheet catalyst is developed for NO3−RR, which exhibits an impressive performance for selective NH3 production at high current density and long-term stability.
This study was published in Angewandte Chemie International Edition on April 29.
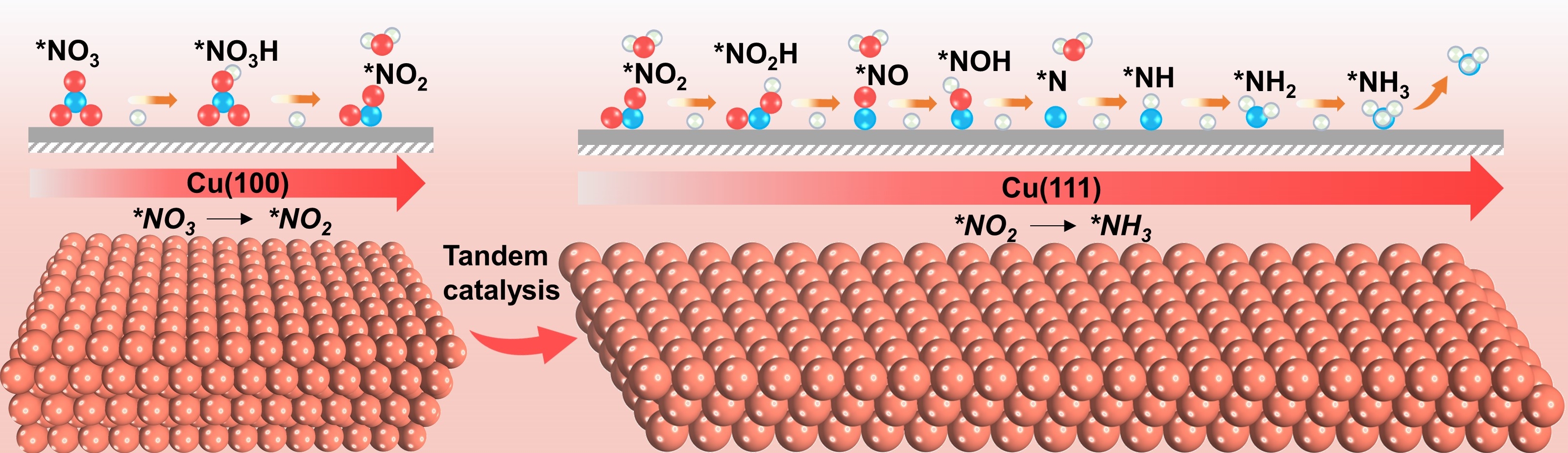
Enhancing electrochemical NO3−RR over Cu nanosheets via facet tandem catalysis (Image by Fu Yunfan and Wang Shuo)
The researchers developed Cu nanosheets in situ derived from CuO nanosheets to catalyze NO3−RR for NH3 production. The Cu nanosheets catalyst exhibits a NH3 partial current density of 665 mA cm−2 and NH3 yield rate of 1.41 mmol h−1cm−2 at −0.59 V vs. reversible hydrogen electrode (RHE). In situ X-ray absorption spectroscopy (XAS) and quasi in situ X-ray photoelectron spectroscopy (XPS) results indicate that the as-prepared CuO nanosheets were electrochemically reduced to metallic Cu as the active site under NO3−RR conditions.
Electrochemical studies and density functional theory calculations suggested that the excellent NO3−RR performance was attributed to the tandem interaction of the Cu(100) and Cu(111) facets. The Cu(100) facets are favorable for NO3− adsorption and conversion into NO2−, followed by the promoted hydrogenation of *NO into *NOH on Cu(111) facets, thus facilitating NH3 production.
This work presents an efficient strategy for enhancing NO3−RR to NH3 production and deepens the reaction mechanism understanding of NO3−RR to NH3 over Cu catalysts.
The above work was supported by the National Key R&D Program of China and the National Natural Science Foundation of China. (Text/Picture Fu Yunfan and Wang Shuo).