Hydrogen spillover, the process where hydrogen atoms activated on a metal surface migrate onto an adjacent support material, is a pivotal yet not fully understood step in many catalytic hydrogenation reactions. Yet, questions remain about how metal/oxide interfacial structures regulate hydrogen spillover and hydrogenation reaction. Understanding these mechanisms is essential for designing efficient catalysts for hydrogenation reactions.
Addressing this challenge, a research team led by Professors FU Qiang and MU Rentao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has made progress in the real-time visualization and enhancement of hydrogen spillover for CO2 hydrogenation. The key discovery is that an inverse catalyst structure, where oxide nanoislands are grown on a metal base, dramatically promotes hydrogen spillover, leading to more efficient CO2 hydrogenation reaction.
This research was published on August 25, 2025, in Angewandte Chemie International Edition.
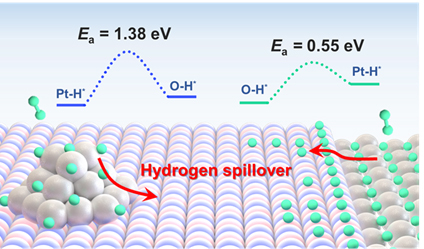
In this work, the researchers constructed two model systems: a Mn3O4/Pt(111) inverse catalyst and a conventional Pt/Mn3O4 catalyst. Using high-pressure scanning tunneling microscopy (STM), they directly imaged the hydrogen spillover behavior and demonstrated that the inverse structure is far more effective at facilitating hydrogen migration. Leveraging this insight, the team synthesized a practical nanocatalyst by depositing MnOₓ onto Pt nanoparticles supported on carbon (MnOx/Pt/C). When tested for CO2 hydrogenation, this inverse catalyst exhibited a CO2 conversion rate 1.8 times higher than that of its traditional Pt/MnOx/C counterpart. These findings reveal that the inverse structure provides unique electronic and geometric advantages at the metal-oxide interface, creating an optimal environment for efficient hydrogen spillover and subsequent hydrogenation reactions.
The work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and Photon Science Center for Carbon Neutrality.
Article link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202515905